After the capsules are manufactured, they are subjected to rigorous quality control tests to ensure that only high-quality capsules reach the customers. Capsule defects, such as cracking, breaking of capsules in the package, and rupture of capsules causing spillage of the filled material, not only affect dosage integrity, but can also severely damage the manufacturer’s reputation. Thus, defects can be avoided by subjecting the capsules to tests that will evaluate the strength of the capsules to withstand forces. Certain tests, such as compression and tension tests, allow the quantification of forces. For these tests, it is essential to vary the force applied. The best results are obtained when the applied force profile represents the conditions that the capsules are most likely to experience.
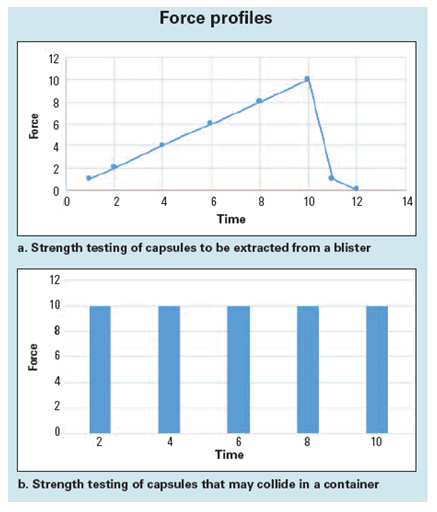
Two different approaches to force testing are presented in Figure 1. Figure 1(a) shows the graph of force applied to a capsule when it is extracted from a blister. Figure 1(b) shows the force applied when the capsules crash into one another in a container.
The mechanical properties of the capsules can be evaluated using a non-destructive method, such as the measurement of the refractive index (RI). As the light passes through a change in medium, it is reflected, and the degree of reflection depends upon the properties of the medium. By the same principle, any change in the RI of the gelatin capsules can predict changes in its mechanical properties. As shown in Figure 2, as the relative humidity (RH) of the storage conditions increases, the RI of the gelatin capsules, which is related to the susceptibility of the capsules to breakage, increases.
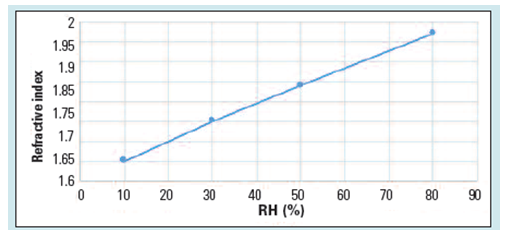
Figure 2: RI of gelatin capsules exposed to different RH. (Adapted from reference 1)
-
Son Y, et al. Refractive index as a surrogate non-destructive measure of capsule brittleness. Respiratory Drug Delivery. 2014;2, 493–496.

