To investigate the brittleness of gelatin capsules, it is imperative to thoroughly characterize the base gelatin polymer. As a degradation product of collagen – a protein abundant in skin, bones, cartilage, and tendons – gelatin has many similarities to collagen in terms of amino acid composition, structure, and certain physicochemical properties. However, with regard to the properties typical of a polymer, gelatin is strongly different to collagen.
At specific conditions of temperature, solvent, and pH, gelatin exhibits different properties owing to its different structural conformation. At normal temperatures, gelatin exists in a collagen-like helical conformation, which is the preferred form for use in capsule preparation. As the temperature increases, gelatin adopts a coiled conformation. This form of gelatin is not favorable because in this state, gelatin exists as a typical rigid-chain polymer and behaves as a brittle material owing to the absence of water.i
The brittle behavior of gelatin changes according to its type. The following two parameters help to assess the brittleness of each type of gelatin.
- Glass transition temperature (Tg): This is the temperature at which the polymer changes from an elastic material to a brittle one owing to changes in chain mobility ii
- Polymer crystallinity: Gelatin coexists in two states, crystalline and amorphous. The crystalline form is a molecular aggregate state that follows a defined pattern, whereas the amorphous form has no defined shape. As with other polymers, the crystalline form of gelatin is brittle.
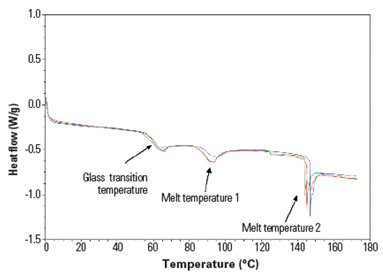
The behaviors of gelatin are best evaluated by using a thermoanalytical technique known as differential scanning calorimetry (DSC). This technique measures the amount of heat required to increase the temperature of the sample material and the reference material as a function of temperature. DSC operates on the principle that when a sample undergoes a physical transformation, such as a phase transition, a different amount of heat is required by the sample than the reference material to maintain both the materials at the same temperature. The DSC graph for gelatin is shown in Figure 4.
Figure 1: DSC for gelatin (Source: unpublished ACG study)
The graph has three prominent features:
- Tg, at which the sample undergoes a change in heat capacity.
- Crystallization (melt temperature 1 in the figure), at which the molecules obtain the freedom of motion to spontaneously arrange themselves into a crystalline form. This peak can be used to confirm that crystallization occurs in the sample, and the area under the curve provides information about the quantity of this phase.
- The melting point (melt temperature 2), above which polymer chains can move around freely.
At ACG, we conducted a study using two gelatin samples, Gelatin 6 and Gelatin 15, which have different degrees of brittleness. The thermal properties of the capsules made with Gelatin 6 and Gelatin 15 were analyzed using DSC.
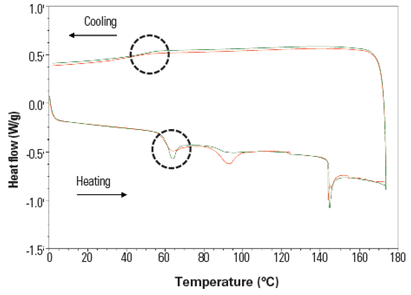
Figure 2: DSC for reference compounds Gelatin 6 (green) and Gelatin 15 (red); X-axis, temperature in °C(Source: Unpublished ACG study)
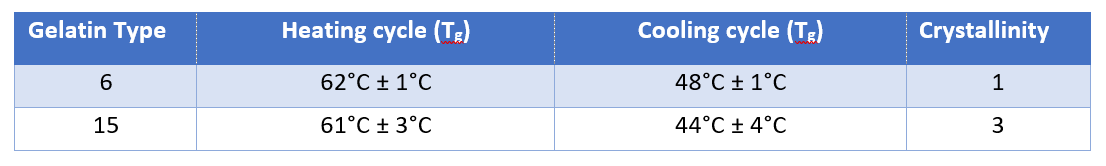
Table 1: Results for both types of gelatin. Confidence interval calculated from Student’s t-distribution for the probability of 95% and N=3. Crystallinity in relative units.
Gelatin 15 has a slightly lower Tg but is less homogenous and has a crystallinity three times higher than that of Gelatin 6. Therefore, Gelatin 6 is a more suitable option, owing to its better mechanical properties.
Taking into consideration the link between the structural conformation of gelatin and the degree of brittleness, it is essential to consider in more detail the manufacturing conditions of gelatin, which can subsequently affect the capsules and capsule filling. In our next blog entry, we will discuss the other factors that affect the brittleness of gelatin capsules.