The number of potential drug candidates has increased dramatically in the past few years, thanks to the technical innovations in the drug discovery area. Highly advanced techniques such as receptor mapping and molecular modelling along with high throughput screening (HTS) assays are contributing significantly to increase the pool of new chemical entities (NCEs) at an exponential rate.
However, the journey from NCE to an approved drug is interlaced with numerous challenges, and one of them is the low water-solubility of NCEs. It is estimated that about 40% of new molecules are poorly water soluble, making the next stages of drug development difficult. This problem gets even more critical when some of these NCEs clear the clinical trials successfully and are ready to move for commercial production. At this stage, the formulation scientists must devise new methods to enhance the water solubility of the NCE to improve bioavailability and facilitate commercial production.
How is drug solubility determined?
Pharmacopoeias categorise the drug from freely water soluble to water insoluble depending upon their equilibrium solubility in water. The most commonly used criterion for categorising drugs based on their solubility and permeability is the bio classification system (BCS). It plays a crucial role in determining the formulation type and processing strategies of oral drug products.
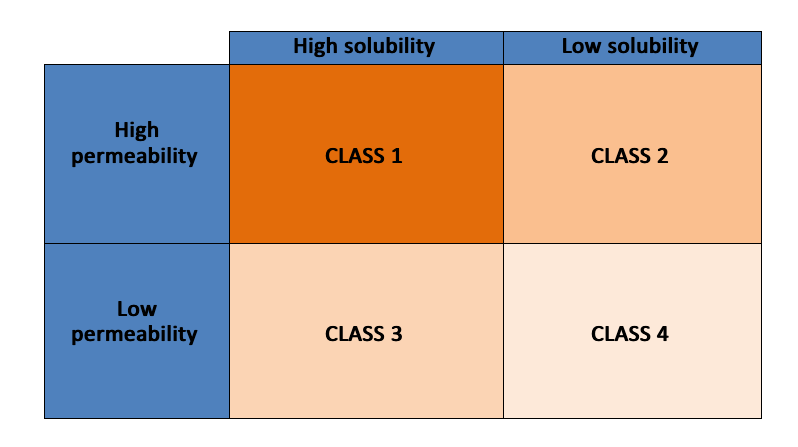
BCS criteria for drug solubility and permeability
The conventional granulation methods don’t help much in improving the solubility of the poorly water-soluble drugs. In some instances, surfactants have been used to enhance the drug solubility and facilitate the subsequent manufacturing processes. However, as drug solubility decreases further (≤ 10 µg/mL), new granulation approaches are required to produce drug products of enhanced solubility and bioavailability. Techniques such as solvent-based spray drying, thermal dry granulation processing, including hot melt extrusion, spray congealing, or spray freezing are being used successfully to produce drug products with better solubility and bioavailability.
Different techniques used for improving solubility
1. Particle size reduction
This method is based on the Noyes-Whitney equation that describes a direct relationship between the dissolution rate and the solid surface area.
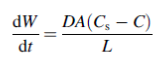
Where dW = the dissolution rate
dt
A = the surface area of the solid
C = concentration of the solid in the diffusion layer surrounding the solid
D = diffusion coefficient
L = diffusion layer thickness
As the particle size decreases, the specific surface area of the drug increases leading to increased solubility. Comminution processes such as jet milling and ball milling can produce drugs with a particle size as small as 1 µm. Drugs like griseofulvin and spironolactone have shown improved drug solubility after micronization. For highly insoluble drugs, nanoparticle drug delivery technologies and supercritical fluids have been used to achieve particle size in the range of 100 nm.
Nanoparticles
Though conventional methods are economical, safe, and effective for particle size reduction, there are several limitations:
- Shear and/or temperature-sensitive drugs may get degraded during the process.
- Drugs with low melting temperatures or thermoplastic properties are difficult to process as size reduction occurs through particle fracture.
- It does not improve the drug dissolution rate of water-insoluble drugs (<10 µg/mL) even after particle size reduction.
Most of these limitations can be overcome by nanoparticle technology. The size of nanoparticles is usually ≤1 µm in one dimension. Nanoparticles also include nanocrystals which are single crystals. In pharmaceutical applications, nanoparticles in the range of 10 -100 nm are used.
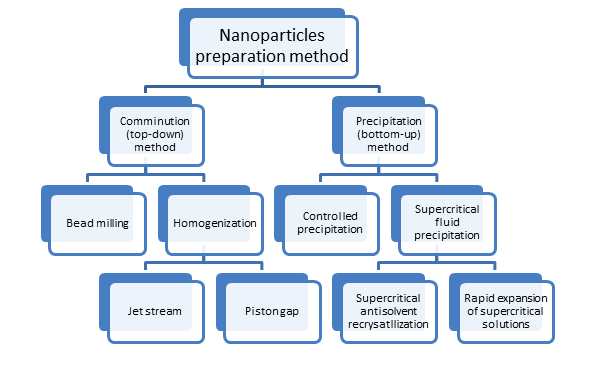
Different nanoparticles preparation methods
Comminution methods
Bead milling – In this equipment, small size beads are used as milling media to prepare nanoparticles. The beads are usually made of yttria zirconiumꟷa strong ceramic, which avoids metal contamination of the nanoparticles during the milling process. The milling chamber is charged with beads, the dispersion medium, drug, and stabilisers. Wet milling provides smaller size nanoparticles than dry bead milling. Water is used as a dispersion medium for poorly soluble drugs. To form nanoparticles from nanosuspensions, surfactants and stabilisers must be used during the milling process.
Bead milling is advantageous in many ways because it is:
- Less expensive
- Relatively simple to use
- Available at R&D scale
- Readily scalable for commercial production
Homogenization – It is another top-down method and can be accomplished by two different techniques.
- Jet stream homogenizer – It involves a collision of two fluid streams under high pressure. The resulting particle collision, shear, and cavitation forces cause the disintegration of drug particles leading to the formation of nanoparticles. A surfactant is required for nanoparticle stabilisation.
The major limitations of jet stream homogenizer are:
– Minimum size
– A high number of cycles are required to achieve a homogenous size distribution
- Piston gap homogenization – In this method, a poorly soluble drug is dispersed in water and passed through a narrow gap using a piston that generates pressure up to 4000 bar. Surfactants are used to facilitate size reduction and stabilise nanoparticles from ripening effects.
Precipitation methods
Controlled precipitation – In this, the drug particles are built from the solution under a controlled condition. The drug is dissolved in a solvent, and the drug solution is then added in a controlled manner to a drug antisolvent under high agitation. This process causes rapid precipitation of drug, generating a large number of nucleation sites and controlling the subsequent growth.
The precipitation method has an advantage over the top-down processing method in that heterogeneous materials can be processed to form cocrystals or coprecipitates, which can further improve the solubility of the poorly soluble drug.
Supercritical fluid (SCF) precipitation – In this technique, carbon dioxide is the most commonly used SCF used to form nanoparticles. Following two processes are widely used in the SCF precipitation technique:
- Supercritical antisolvent recrystallization (SAS) – In this method, the drug is solubilised in a solvent and then sprayed into SCF. The drug must be insoluble in SCF, while the organic solvent must be miscible in SCF. The SCF diffuses into the drug-solvent droplets, and the miscible solvent expands with the SCF causing drug precipitation. SAS method is more flexible because it enables the control of particle growth through the solvent selection and solvent extraction conditions of SCF.
- Rapid expansion of supercritical solutions (RESS) – This method is suitable for drugs soluble in SCF. The drug or a drug-polymer mixture is dissolved in SCF, and the SCF solution is then sprayed into a lower pressure vessel. The rapid expansion of the solution reduces the density of the SCF and supersaturates the drug or drug polymer in the lower-pressure solution. This results in the precipitation of drug or drug-polymer particles of reduced size and narrow size distribution. Major drawbacks of the RESS method are that only a few drugs are soluble in SCF, and process throughput rates for precipitates are slow.
2. Drug complexation
Drug complexes are entities in which drug molecules are bound to other molecules of complexing agent or ions via noncovalent bonds such as hydrogen bond or Vander Waals bond. Certain drug complexes are more water soluble than the initial drug molecules, and hence, it is an effective way to increase drug solubility and bioavailability. For drug complexation, all the components of the complex must be pharmaceutically acceptable. Drug complexes can be formed either using hydrophilic polymers or cyclodextrins (CDs).
Drug complexes can be formed with
- Hydrophilic polymers such as PVP, HPMC or HPC, crospovidone
- Cyclodextrins such as α-, β– and γ-cyclodextrin
3. Solid dispersions
Solid dispersions are molecular or near-molecular mixtures of drugs and hydrophilic excipients. Enhanced solubility is achieved because of the even dispersion of the drug in an inert biological matrix at a solid state prepared by melting, solvent, or melting-solvent method. The inert carrier can be:
- Water-soluble polymers for poorly water-soluble active (e.g., PEG, PVP, PVP/VA, HPMC)
- Water-insoluble polymers for modified release (for example, ethylcellulose, HPMCP, polyacrylates, polymethacrylates)
Solid dispersions enhance dissolution rate and solubility of poorly water-soluble drugs by size reduction, improving wettability, and amorphous transformation.
The way forward
The water solubility of a drug is a critical factor in determining the therapeutic activity of the formulation. Although there are various methods available to improve the drug water solubility, the final decision rests with the formulation scientists to choose the technique that will optimise the formulation and at the same time will be feasible for commercial production.
Reference
Handbook of pharmaceutical granulation technology, Edited by Dilip Parikh, 3rd edition

