The concept of modified–release (MR) dosage forms materialised as a part of the product lifecycle management strategy. The drying pipeline of new molecules compelled pharmaceutical companies to develop new approaches to retain the exclusivity of patented molecules and gain maximum commercial benefits. This strategy became a colossal success setting many companies on the path where they are now considering the MR approach right at the beginning of the drug development stage.
From a therapeutic perspective, the altered release profile of a drug product does provide a plethora of benefits to the patients.
- It offers an opportunity to modulate the drug concentration in the body to provide a sustained therapeutic effect over a period.
- Since MR dosages are required to be taken once a day (in most cases), this reduces the dosing frequency significantly, resulting in better patient compliance.
- An MR dosage form maintains a steady-state drug plasma concentration in the body, thus avoiding side-effects related to fluctuations in drug concentration.
- The technique allows controlling the site of drug delivery within the GIT.
Targeted product profile (TPP)
A successful formulation of MR dosage form begins with a well-defined TPP, and it is based on the following parameters:
- Establishing a dose range of the molecule that is therapeutically active
- Desired dosing frequency
- The desired release profile of the drug (zero-order release or pulsatile release) along with the minimum and maximum drug plasma levels that are within the therapeutic window
- Dosage form
Formulation considerations
- Active pharmaceutical ingredient (API) – Granulated MR (GMR) dosage form is not possible for every drug molecule. The API must be assessed for the suitability of preparing an MR formulation based on the following two characteristics:
- The Biopharmaceutical Classification System (BCS) category of a drug molecule gives more information about its solubility and permeability behaviour. An API with good solubility and permeability can dissolve in the body fluids and pass through the biological membranes at the absorption site.
- The dose-solubility ratio of an API helps in selecting a suitable MR technology.
- Release modifying ingredient – After API, it is the second most crucial ingredient of GMR formulation that helps to achieve the modified drug release profile. The following two types of materials are typically used as release modifiers.
- Polymers – These are the most commonly used materials for modifying the drug release profile. Depending upon the physicochemical and biopharmaceutical properties of the drug and the process requirement, either a polymer or a mixture of polymers can be used in the formulation. Generally, high viscosity grades of polymers are used. Following parameters must be taken into consideration before selecting a polymer for a GMR formulation:
- The polymer must have reproducible physical properties.
- The polymer should not interfere with the drug’s therapeutic effect.
- The polymer must be chemically compatible with the API.
- Long-chain hydrocarbons – The hydrophobic properties of long-chain hydrocarbons assist in modifying the drug release from the GMR. Examples include wax and fat materials such as carnauba wax, glyceryl behenate, and glyceryl palmitostearate.
Apart from these two, other ingredients can be added to the formulation depending upon the granulation method used for the manufacturing. All the ingredients selected must be compatible with each other.
Dosage performance factors
The modified drug release pattern is the sole factor that distinguishes MR dosage forms from the immediate-release ones. Naturally, it’s essential to have adequate knowledge of the drug release mechanism and the associated factors before the formulation scientists start working on the formulation.
Drug release mechanism – There are various drug release mechanisms from the GMR, such as diffusion, swelling, erosion, and dissolution. It can be a single mechanism or a combination of these. A thorough understanding of the release mechanism helps the formulation scientists to design a formulation with the desired TPP. The nature of the polymer and its behaviour in the release medium also contribute to the drug release mechanism.
Drug release pattern and predictability – The release of drug molecules from the dosage form can be described by different mathematical equations. One such equation known as Fick’s first law describes the diffusion of drug molecules. It relates the amount of material flowing through a unit cross–section as follows:
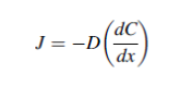
Where J = diffusion flux
D = diffusion coefficient or diffusivity
C = concentration
X = distance of movement
With the knowledge of the drug-release mechanism and the available mathematical equations, it becomes easy for the formulation scientists to convert the drug-release process from the dosage form into a theoretical model and understand its kinetics.
Reproducibility of drug-release pattern – The drug-release pattern of an MR dosage form is evaluated by using in–vitro dissolution methods as defined by regulatory guidelines. For a satisfactory performance of an MR dosage form, the guidelines recommend a minimum of three dissolution time points:
- An early time point to ensure that there is no dose dumping
- A middle time point for control of release profile
- The last time point to ensure that at least 80% of drug release has occurred
Besides, the following two factors must be considered during dissolution testing of an MR dosage form.
- In vitro-in vivo correlation (IVIVC) – It is a predictive mathematical model that describes a relationship between an in vitro property of a dosage form (e.g., drug dissolution profile) to an in vivo response elicited by the same dosage form (e.g., drug’s extent of absorption). Establishing an IVIVC significantly cuts down the number of bioavailability/bioequivalence studies needed at the initial development and approval process.
- Dose dumping – Ideally, the drug-release from an MR dosage form should not be affected by the presence of food and alcohol. This becomes particularly important for drugs with a narrow therapeutic index where a complete release of dose before the intended time and place can lead to potentially toxic drug levels in the body. An in vitro dissolution study can address this issue.
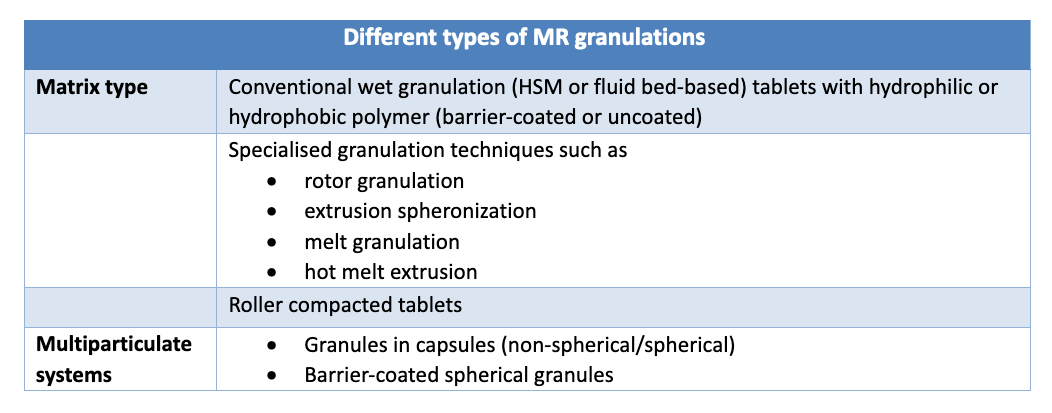
Different approaches used for GMR processing
- A conventional tablet with an immediate release profile can be converted into an MR dosage form by adding a drug-release retarding polymer in the formulation.
- A foam binder technology can be used in which a foamed binder solution is directly added to the dry powders in the granulator.
- For drugs with a short half-life, MR formulation can be prepared in the form of bilayer tablets. One layer of this tablet immediately releases the drug (about 60%) to achieve adequate plasma levels. The other layer releases the rest of the drug at a slower rate to maintain the effective drug concentration in the body for a longer time.
- Spray drying has been used for the preparation of MR dosage forms. In this, a solution or suspension of the drug is pumped and atomised into a drying chamber under a controlled airflow to produce granules.
Takeaway
Although bringing a granulated MR dosage form on the market offers various commercial benefits to the pharma companies, developing such a formulation is quite a task, calling for multiple iterations and considerations of the key materials, analytical, and manufacturing processes. However, looking at the current granulation techniques and the rapid advances in this area, it is safe to assume that developing a granulated MR dosage form won’t be a challenge anymore in the near future.

