In the past few years, the number of orphan drugs approved by the FDA has gone steadily up and is still rising. According to Evaluate Pharma‘s report, orphan drugs are expected to make one–fifth of worldwide prescription sales, which amounts to $242 billion by 2024. In fact, orphan drug sales across the world are estimated to grow at a CAGR of 12.3% from 2019 to 2024. This rate is nearly double the forecast rate for the non-orphan drug market.
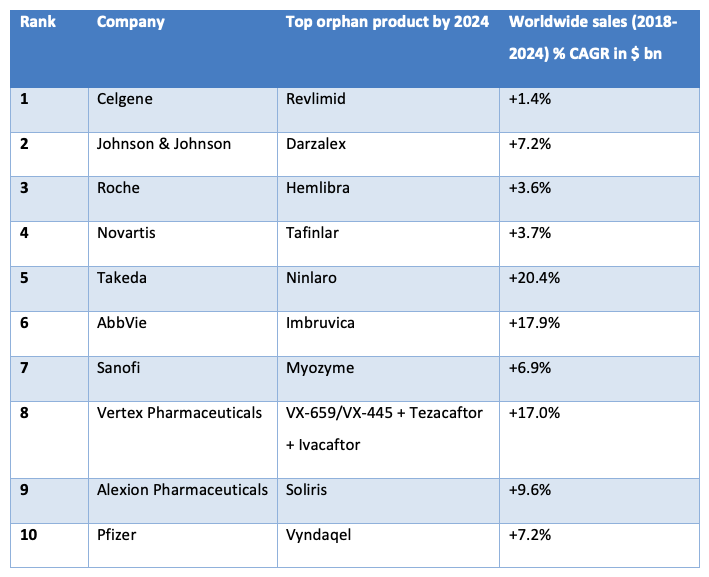
Top 10 companies with their lead product and sales forecast for 2018-24
(Source: Orphan Drug Report 2019, 6th Edition)
The catalysts
Before we dive into the subject of orphan drugs, let‘s first understand what these are. Orphan drugs are the drugs intended to treat either a rare disease (orphan disease) or a more common disease threatening public health and manufactured on a non-profit basis by pharma manufacturers. For example, drugs and vaccines used for tropical diseases. A drug might also have an “orphan“ indication where the drug has been used frequently to treat one disease but may not have been tested for another rare medical condition.
Currently, many big pharma companies are opting in for orphan drug development. This change has not happened overnight but is the result of the various incentive program initiated by the FDA and the changing pharma market scenarios.
Benefits to pharma companies
The cut-throat competition of the pharma market has reached its high because of multiple reasons. Several blockbuster drugs are on the verge of losing the patent exclusivity cutting Big pharma’s revenues. Generic drugs are flooding the market every day because of the rising number of generic manufacturers and the patent expired drugs. The drug discovery programs are running low because of the lack of new potential molecules, and the cost of the development process is taking a heavy toll on the pharma companies. On top of all this, the FDA guidelines are becoming more stringent compelling the pharma manufacturers to maintain high standards and quality throughout the manufacturing, leaving a little room for revenues. In such a scenario, orphan drugs are proving to be beacons for the pharma companies as they can help reduce the impact of revenue loss due to the patent expiry of blockbuster drugs. Working on orphan drugs can also open up numerous possibilities of new therapeutic areas, diagnosis, treatment, monitoring, and patient support, thus creating an opportunity for pharma companies to offer an integrated healthcare solution.
FDA‘s interest
Orphan diseases or rare diseases manifest in a maximum of about 6-8% of the world population. Almost more than 50% of orphan diseases appear during adulthood. At present, approximately 7000 rare diseases have been listed, and 80% have been traced back to genetic origins. The development of orphan drugs can throw light on the pathophysiology of the rare diseases helping to understand their genetic basis, which in turn can help to develop an effective treatment for this small group of population. To trigger the research in this area, the Orphan Drugs Act was established in the US in 1983, and it did succeed in motivating the pharma companies. Before this act, only 38 orphan drugs were approved, whereas, in 2019, the total number of orphan drugs approved increased to 600. Other countries also followed suit, adopting the legislation; notable among these are Japan (1993) and the European Union (2000). Besides the act, the FDA has offered various benefits to the pharma companies working on molecules with orphan drug designation. These include reduced or wavered regulatory fees, the opportunity for tax credits, and subsidies for clinical trials. Also, the orphan drug designation provides seven years of market exclusivity in the US, whereas, in the EU, it is ten years for the approved product.
The obstacles
Despite incentivisation by the FDA, developing an orphan drug is not an easy task. The rare occurrence of the disease and the population creates many challenges along the road.
- Lack of pathophysiology – When it comes to understanding the underlying pathology and natural history of the orphan disease, research scientists are still in the dark and struggling to find out more about it.
- Small patient pool – The rare occurrence of the disease makes it difficult to understand the patient population’s fundamental aspects. Further, the small pool of patients who are often heterogeneous, ill-defined, and spread over large geographical areas create challenges in identifying the right patients for the clinical trials.
- Lack of animal model – As there is no fundamental knowledge on the epidemiology and pathophysiology of rare diseases, animal models cannot be developed. This makes it challenging to identify the most relevant clinical endpoints to use in clinical trials.
- Lack of skill set – Developing an orphan drug requires an entirely different approach with a different skill set. The design of clinical trials differs significantly from that of non-orphan drugs, requiring an adaptive process and collaboration with various stakeholders, including specialised centres, regulators, patient advocacy groups, and experienced CROs. Since about 50% of rare diseases manifest in the paediatric population, using a placebo in trials involving children presents ethical issues and controversies.
- Cost intensive – The affordability of treatment with orphan drugs is perhaps the biggest hurdle for the patients and a tricky aspect with different stakeholders.
The way forward
There is no denying that the rising number of approved orphan drugs has opened up new avenues for the pharma companies in terms of revenues and business model. But more than that, it has given hope to those patients who till now had only a few or no drugs available for the treatment. Although the cost of such treatment is a disputable matter, we can hope that appropriate government policies and the pharma companies‘ sympathetic approach towards patient support programs can help the maximum number of patients access these drugs quickly and easily.
References:
-
https://www.evaluate.com/sites/default/files/media/download-files/EvaluatePharma_Orphan_Drug_Report_2019.pdf
-
https://www.labiotech.eu/partner/orphan-drugs-development-future/
-
https://www.raps.org/news-and-articles/news-articles/2020/6/fda-officials-update-on-orphan-drugs-gene-therapie
-
https://www.fda.gov/news-events/fda-voices/innovation-new-drug-approvals-2019-advances-patient-care-across-broad-range-diseases
-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996062/



